- Saturday
- April 20th, 2024
- Submit Post

[ad_1] On Friday morning, Iranian air defences shot down three drones above its central province of Isfahan. Iran has yet to announce the results of its investigation into the incident, but the US said early on that Israel launched the...

[ad_1] <!-- --> KGBV in-charge special officer Vanitha said 11 students complained of stomachache and diarrhoea after dinner at 10 pm. Updated On - 20 April 2024, 11:57 AM
KGBV in-charge special officer Vanitha said 11 students...

[ad_1] A digitally empowered ecosystem, enabling the complete cycle of agricultural processes - from sowing to farming to supply chain to post-harvest logistics - is what constitutes the “digitally unified agri value chain”. Comprising different stakeholders, from farmers and aggregators...

[ad_1] It is not easy writing historical fiction. Histories are often contested, pivotal points in time viewed from entirely different loyalties lead to entirely different perspectives. It is almost an impossible task to weave these disparate threads into a single...
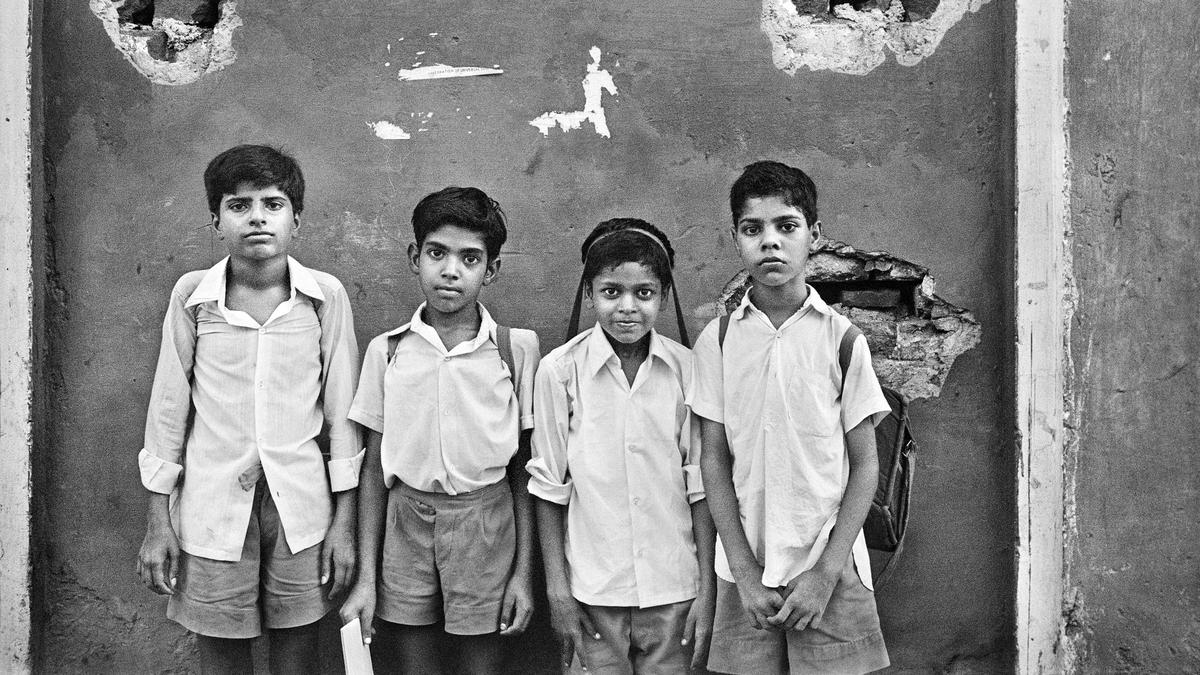
[ad_1] âAt no given time am I without a camera,â asserts Raghu Rai, one of Indiaâs most important photographers, who is the subject of a major exhibition at the Kiran Nadar Museum of Art in New Delhi. Now 81, Rai...

[ad_1] What's your money worth? A series from the front lines of the cost-of-living crisis, where people who have been hit hard share their monthly expenses. Name: Manisha Santosh Kadam Age: 42 Born: Manchar, in the Indian state of Maharashtra...

[ad_1] <!-- --> I'm referring to individuals orchestrating large-scale bot spam operations that undoubtedly degrade content quality," replied the billionaire to a follower Published Date - 20 April 2024, 10:30 AM
New Delhi: Some people are...

[ad_1] Tata Group may strike a deal to take control of Pegatron Corp.âs iPhone manufacturing operations in India as soon as May, cementing Apple Inc.âs relationship with one of the countryâs most influential conglomerates.Tata Group is in the final stages...

[ad_1] In the last section of Salman Rushdie’s autobiographical nonfiction, Knife, there’s a startling piece of information regarding the man who attempted to murder the writer on August 12, 2022, at the Chautauqua Amphitheatre in New York. Rushdie does not...
 Loading posts...
Loading posts...  All posts loaded - Crime Today News
All posts loaded - Crime Today News
No more posts
